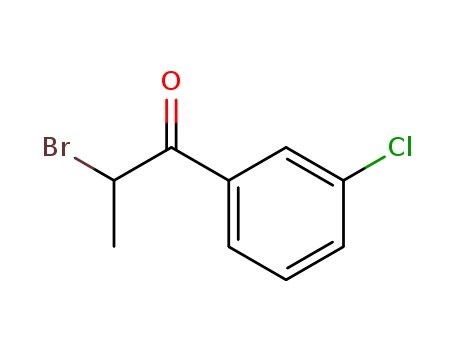
34911-51-8
- Product Name:2-Bromo-3'-chloropropiophenone
- Molecular Formula:C9H8BrClO
- Purity:99%
- Molecular Weight:247.519
Product Details;
CasNo: 34911-51-8
Molecular Formula: C9H8BrClO
Appearance: Yellow to brown colored liquid
2-Bromo-3'-chloropropiophenone is used as an important intermediate for raw material and intermediate used in organic synthesis agrochemical, pharmaceutical and dyestuff field.
Buy High Quality Best Quality 2-Bromo-3'-chloropropiophenone 34911-51-8 On Stock
- Molecular Formula:C9H8BrClO
- Molecular Weight:247.519
- Appearance/Colour:Yellow to brown colored liquid
- Refractive Index:1.5770
- Boiling Point:295 °C at 760 mmHg
- Flash Point:132.2 °C
- PSA:17.07000
- Density:1.518 g/cm3
- LogP:3.30610
34911-51-8 Relevant articles
Microwave-assisted Vilsmeier-Haack synthesis of Pyrazole-4-carbaldehydes
Kumari, Poonam,Sood, Sumit,Kumar, Anil,Singh, Karan
, p. 796 - 804 (2019/11/28)
The synthesis of 4-formylpyrazoles using...
A Method for the Catalytic Enantioselective Synthesis of Chiral α-Azido and α-Amino Ketones from Racemic α-Bromo Ketones, and Its Generalization to the Formation of Bonds to C, O, and S
Da Silva Gomes, Roberto,Corey
supporting information, p. 20058 - 20061 (2019/12/27)
A new and practical method has been deve...
Novel benzene-based carbamates for ache/bche inhibition: Synthesis and ligand/structure-oriented sar study
Bak, Andrzej,Kozik, Violetta,Kozakiewicz, Dariusz,Gajcy, Kamila,Strub, Daniel Jan,Swietlicka, Aleksandra,Stepankova, Sarka,Imramovsky, Ales,Polanski, Jaroslaw,Smolinski, Adam,Jampilek, Josef
, (2019/05/10)
A series of new benzene-based derivative...
Synthesis of α,β-dibromo ketones by photolysis of α-bromo ketones with N-bromosuccinimide: Photoinduced β-bromination of α-bromo ketones
Moon, Da Yoon,An, Sejin,Park, Bong Ser
, (2019/10/28)
Irradiation of α-bromopropiophenones in ...
34911-51-8 Process route
-
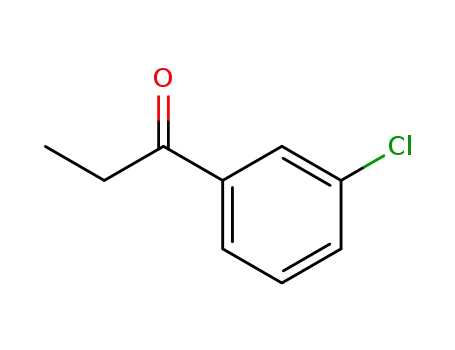
- 34841-35-5
3'-chloro-propiophenone

-
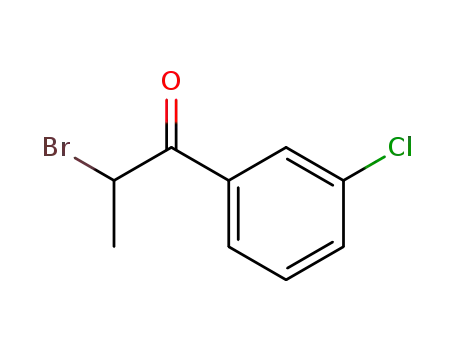
- 34911-51-8
2-bromo-3'-chloropropiophenone
| Conditions | Yield |
|---|---|
|
With bromine; at 20 ℃; for 0.333333h;
|
100% |
|
With 1,4-dioxane dibromide; In diethyl ether; at 20 ℃; for 1h;
|
100% |
|
With bromine; In dichloromethane; at 20 ℃; for 1h;
|
100% |
|
With bromine; In acetonitrile; at 0 - 30 ℃; for 1.5h;
|
100% |
|
With bromine; In dichloromethane; at 44 ℃; under 3878.71 Torr; Flow reactor;
|
99% |
|
With bromine; In dichloromethane; for 16h; Inert atmosphere;
|
95% |
|
With bromine; In dichloromethane; for 16h; Inert atmosphere;
|
95% |
|
With bromine; In diethyl ether; for 0.5h; Inert atmosphere;
|
94% |
|
With hydrogen bromide; potassium iodide; sodium nitrite; In water; acetonitrile; at 0 - 20 ℃; for 10h;
|
85% |
|
With trifluorormethanesulfonic acid; bromine; In dichloromethane; at 20 ℃; Inert atmosphere;
|
79% |
|
With bromine; In chloroform; at 20 ℃;
|
60% |
|
With bromine; acetic acid;
|
|
|
With bromine; In tetrachloromethane;
|
|
|
With bromine; In dichloromethane; cooling;
|
|
|
With bromine; In dichloromethane; water; at 35 ℃;
|
|
|
With copper(ll) bromide; In ethanol;
|
|
|
With bromine; at 50 - 80 ℃; for 0.5h;
|
|
|
With 1,4-dioxane dibromide; In 1,4-dioxane; water;
|
14.8 g (85%) |
|
With 1,4-dioxane dibromide; In 1,4-dioxane; water;
|
14.8 g (85%) |
|
With bromine; In dichloromethane;
|
|
|
With 1,4-dioxane dibromide; In 1,4-dioxane; water;
|
14.8 g (85%) |
|
With bromine; In water; at 20 - 35 ℃; for 1.5 - 2h;
|
|
|
With bromine; at 60 - 85 ℃; for 2 - 5.5h; Product distribution / selectivity;
|
|
|
With bromine; In 1,2-dichloro-ethane; at 65 ℃; for 5h; Product distribution / selectivity;
|
|
|
With bromine; In dichloromethane;
|
|
|
With bromine; In dichloromethane;
|
|
|
With bromine; In dichloromethane; at 20 ℃; for 2.25h;
|
|
|
With N-Bromosuccinimide; toluene-4-sulfonic acid; at 20 - 65 ℃; neat (no solvent);
|
99.96 %Chromat. |
|
With bromine; In dichloromethane; at 20 ℃; Inert atmosphere;
|
|
|
With bromine; In diethyl ether; at 20 ℃; Inert atmosphere;
|
|
|
With hydrogen bromide; dihydrogen peroxide; In dichloromethane; at 20 - 25 ℃; for 1h; Solvent;
|
49.1 g |
|
With N-Bromosuccinimide; toluene-4-sulfonic acid; In acetonitrile; at 60 ℃; for 4h;
|
|
|
With copper(ll) bromide;
|
|
|
With sulfuric acid; dihydrogen peroxide; sodium bromide; In dichloromethane; water; at 40 ℃; for 2.33333h;
|
|
|
With bromine; In dichloromethane;
|
|
|
With copper(ll) bromide;
|
|
|
With bromine; acetic acid; at 0 - 20 ℃;
|
-
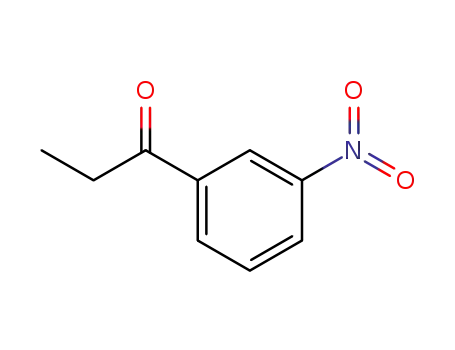
- 17408-16-1
3'-nitropropiophenone

-

- 34911-51-8
2-bromo-3'-chloropropiophenone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: H2 / Pd-C / ethanol
2: NaNO2, Cu2Cl2, HCl
3: Br2, AcOH
With copper(I) chloride; hydrogenchloride; hydrogen; bromine; acetic acid; sodium nitrite; palladium on activated charcoal; In ethanol;
|
34911-51-8 Upstream products
-
34841-35-5

3'-chloro-propiophenone
-
17408-16-1

3'-nitropropiophenone
-
1197-05-3
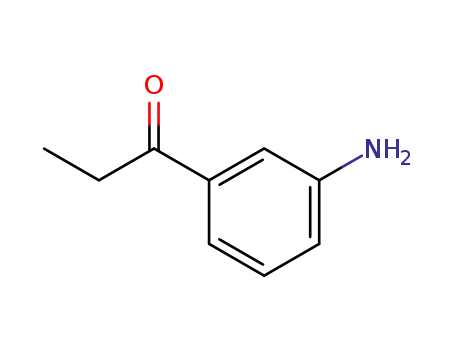
1-(3-amino-phenyl)-propan-1-one
-
93-55-0
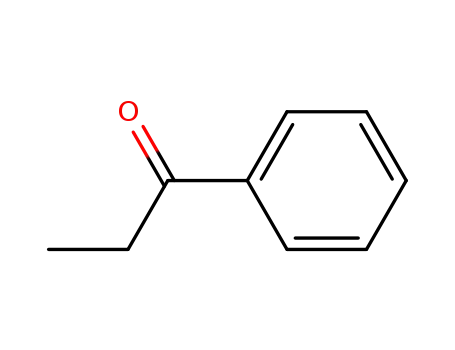
1-phenyl-propan-1-one
34911-51-8 Downstream products
-
94026-07-0
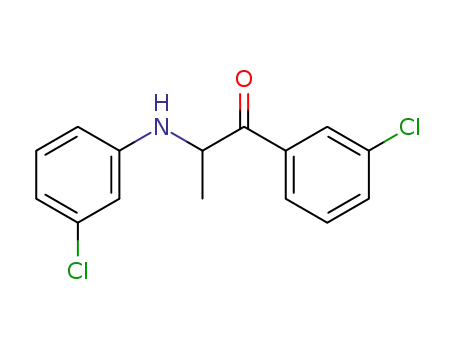
2-<3-Chlor-anilino>-1-<3-chlor-phenyl>-propanon-(1)
-
881384-80-1
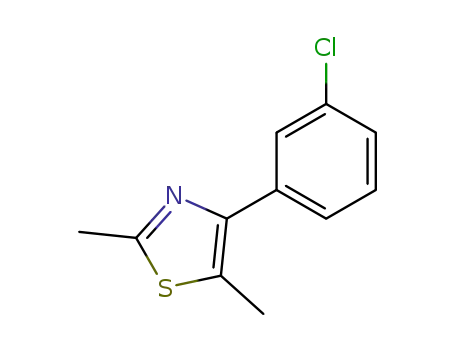
4-(3-chlorophenyl)-2,5-dimethyl-1,3-thiazole
-
913728-76-4
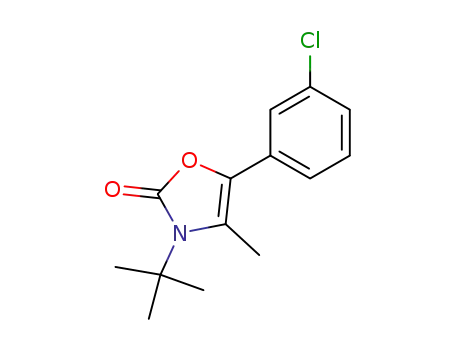
3-tert-butyl-5-(3-chlorophenyl)-4-methyloxazolin-2-one
-
913728-79-7
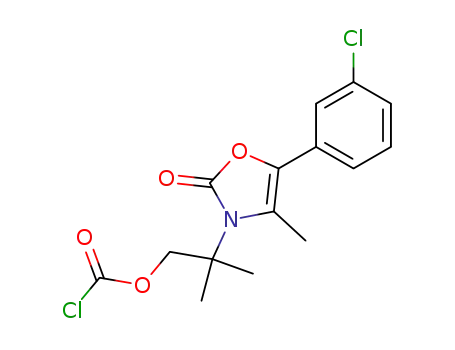
5-(3-chlorophenyl)-3-[2-(chlorocarbonyloxy)-1,1-dimethylethyl]-4-methyloxazolin-2-one
Relevant Products
-
Ethylmagnesium bromide
CAS:925-90-6
-
S-23
CAS:1010396-29-8
-
Copper Chromite
CAS:12053-18-8