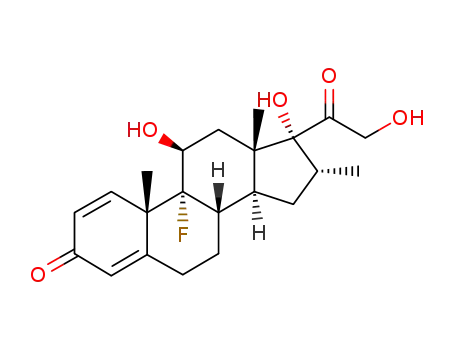
50-02-2
- Product Name:Dexamethasone
- Molecular Formula:C22H29FO5
- Purity:99%
- Molecular Weight:392.468
Product Details;
CasNo: 50-02-2
Molecular Formula: C22H29FO5
Appearance: White crystalline solid
Chinese Manufacturer Supply 99% Pure Dexamethasone 50-02-2 Efficient Delivery
- Molecular Formula:C22H29FO5
- Molecular Weight:392.468
- Appearance/Colour:White crystalline solid
- Vapor Pressure:2.81E-15mmHg at 25°C
- Melting Point:262-264 °C(lit.)
- Refractive Index:76 ° (C=1, Dioxane)
- Boiling Point:568.2 °C at 760 mmHg
- PKA:12.13±0.70(Predicted)
- Flash Point:297.5 °C
- PSA:94.83000
- Density:1.32 g/cm3
- LogP:1.89570
Dexamethasone(Cas 50-02-2) Usage
|
Anti-inflammatory effects |
Dexamethasone reduces and prevents the tissue response to inflammation, thereby reducing the manifestations of inflammation. Hormones inhibit the accumulation of inflammatory cells, including macrophages and leukocytes, at sites of inflammation, and inhibit phagocytosis, the release of lysosomal enzymes, and the synthesis and release of inflammatory chemical mediators. |
|
Description |
Dexamethasone is an Anti-inflammatory glucocorticoid that is used to treat inflammatory and autoimmune conditions such as rheumatoid arthritis and bronchospasm. It is useful to study apoptosis, cell signaling pathways and gene expression. It is associated with marbofloxacin and clotrimazole and finds application in veterinary medicine to treat difficult ear infections in dogs. It is also used to treat horses with swelling of of distal limbs and general bruising in combination with trichlormethiazide. Dexamethasone induces the production of phospholipase A2 inhibitory protein (lipocortin). It also inhibits induction of nitric oxide synthase (IC50=5 nM). Dexamethasone has been shown to cause reduction in cyclin A and Cd k2 activity, inhibition of G1/S transition in osteoblasts and inhibition of phosphorylation of Rb protein in vitro. Dexamethasone has been observed to induce apoptosis in human thymocytes and eosinophils, but inhibits apoptosis in neutrophils in vitro. Dexamethasone is an activator of IDO. Dexamethasone Inhibits the expression of the inducible but not the constitutive nitric oxide synthase in vascular endothelial cells (IC50=5 nM). Dexamethasone regulates T cell survival, growth, and differentiation.Enhances active cation transport in aortic smooth muscle cells by stimulating the Na+-K+ pump. Has anti-inflammatory and anti-rheumatic properties. Induces apoptosis in human thymocytes. In general, 500-1000 nM of dexamethasone is sufficient to induce apoptosis following a 6-hour incubation at 37°C. |
|
Side Effects |
Dexamethasone is a artificially synthetic glucocorticoid, belonging to a long-term glucocorticoid drugs. Glucocorticoids can promote the metabolism of the three major nutrients while preventing protein synthesis with long-term topical being able to causing more serious consequences. However, the adverse effects should be much smaller than oral medication. Common side effects of systemic corticosteroid include: It can cause stomach discomfort and increased sensitivity to stomach ulcers. It can Increase the appetite and results in a significant increase in body weight. Potential patients with diabetes: glucose intolerance in patients with aggravating existing diabetes. It can cause mental illness including personality changes, irritability, agitation, and mania. It can be used for the long-term treatment of osteoporosis: pathological fractures (such as hip). It can cause elevated liver enzymes, fatty liver degeneration (usually reversible). For patients of nephrotic syndrome, applying long-term high-dose medication is likely to cause large side effects such as gastrointestinal ulcers and avascular necrosis. For treatment of nephrotic syndrome, it is better to apply prednisone acetate tablets. Dexamethasone can be used for the treatment of high altitude cerebral edema and pulmonary edema. Upon climbing expeditions, people can apply it to alleviate altitude sickness. Combination with marbofloxacin and clotrimazole, etc. can be used for treating the ear infection and allergies of a dog or a bird. |
|
Drug Reactions |
Dexamethasone is a corticosteroid known as a glucocorticoid. Corticosteroids are meant to resemble a naturally occurring hormone produced in the adrenal cortex, cortisol. Corticosteroids act on the immune system by blocking the production of substances that trigger inflammatory and immune responses. Dexamethasone may react with these drugs: Amphotericin Aspirin Cyclophosphamide Cyclosporine Digoxin Daunorubicin HCl Doxorubicin HCl Insulin Mitotane Phenobarbital Phenytoin sodium Rifampin Rimadyl |
|
Chemical Properties |
White or almost white, crystalline powder. |
|
Originator |
Dexacen,Central,US,1977 |
|
Uses |
Glucocorticoid. |
|
Indications |
Cushing’s disease is defined as hypercortisolism due to chronic overproduction of corticotrophin by a corticotroph adenoma. Cortisol’s lack of suppressibility during the administration of low doses of dexamethasone but suppressibility during high-dose dexamethasone is the key diagnostic finding in 99% of the patients with Cushing’s disease. This contrasts with the lack of glucocorticoid suppressibility typically found in patients with corticotrophin-independent hypercortisolism (Cushing’s syndrome). A judicious selection of the available tests may be necessary to obtain an accurate diagnosis in patients with Cushing’s syndrome. |
|
Manufacturing Process |
The preparation of dexamethasone acetate is described in US Patent 3,007,923 as follows. 1.5 cc of dimethylformamide and 1.5 cc of anhydrous hydrofluoric acid are admixed and treated with 480 mg of 9β,11β-epoxy-17αhydroxy-21-acetoxy-16α-methyl-?1,4-pregnadiene-3,20-dione (prepared according to E.P. Oliveto et al, J. Am. Chem. Soc., 80, 44331, 1958). The steroid dissolves in about 15 minutes. The reaction mixture is shaken for two hours at a temperature between 0 and +5°C, and then poured into 75 cc ofwater containing in suspension, 7.5 grams of sodium bicarbonate. The mixture is vacuum filtered, the filter cake washed and then dried at 100°C, yielding 460 mg of crude hexadecadrol contaminated with a small amount of the starting material. A single recrystallization from methylene chloride yields 370 mg of the pure product having a melting point of 170°C and 229°C. The mother liquor yields 62 mg of the starting material, and a remainder constituting a mixture of starting and final materials with little other contamination. |
|
Brand name |
Aeroseb-Dex (Allergan); Decadron (Merck); Dexone (Solvay Pharmaceuticals); Hexadrol (Organon); Maxidex (Alcon); Mymethasone (Morton Grove). |
|
Therapeutic Function |
9-Fluoro-11β,17-dihydroxy-21-acetoxy-16α-methylpregna1,4-diene-3,20-dione |
|
General Description |
Dexamethasone, 9-fluoro-11β,17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione,is the 16 -isomer of betamethasone.Dexamethasone acetate, USP (21-acetate)Dexamethasone sodium phosphate, USP (21-sodiumphosphate). |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Dexamethasone may be sensitive to prolonged exposure to light. Dexamethasone is incompatible with strong oxidizers, strong acids, acid chlorides and acid anhydrides. Oxidation may occur with bases. |
|
Fire Hazard |
Flash point data for Dexamethasone are not available; however, Dexamethasone is probably combustible. |
|
Biological Activity |
Glucocorticoid; anti-inflammatory. Reduces levels of activated NF- κ B in immature dendritic cells (DCs) and inhibits differentiation into mature DCs. |
|
Biochem/physiol Actions |
Target IC50: 5 nM Inhibiting the expression of inducible but not constitutive nitric oxide synthase in vascular endothelial cells |
|
Pharmacology |
Dexamethasone is a corticosteroid with high glucocorticoid activity and virtually no mineralocorticoid activity. I ts mechanism of action as an antiemetic is unknown, but it is possible that either direct genomic or indirect non-genomic effects on 5-HT3 and GABAA receptors contribute to its antiemetic activity. Many of the original studies were carried out using 8– 10mg of dexamethasone phosphate, but smaller doses (2.5–4mg) provide equal antiemetic efficacy with minimal risk of adverse effects. Concerns relating to adrenal suppression and other steroid-induced adverse effects (including increased risk of bleeding) after a single dose of dexamethasone remain largely unfounded. O ne of the most unpleasant adverse effects of dexamethasone involves intense perineal stimulation after rapid i.v. injection. |
|
Pharmacokinetics |
The activity of dexamethasone, as measured by glycogen deposition, is 20 times greater than that of hydrocortisone. It has five times the anti-inflammatory activity of prednisolone. Clinical data indicate that this compound has seven times the antirheumatic potency of prednisolone. It is roughly 30 times more potent than hydrocortisone. Its pharmacokinetics are presented in Table 33.3. Routes of metabolism for dexamethasone are similar to those for prednisolone, with its primary 6β-hydroxy metabolite being recovered in urine. Dexamethasone sodium phosphate is the water-soluble sodium salt of the 21-phosphate ester, with an IV half-life of less than 10 minutes because of rapid hydrolysis by plasma phosphatases. Peak plasma levels for dexamethasone usually are attained in approximately 10 to 20 minutes following its IV administered dose. A similar reaction occurs when the phosphate ester is applied topically or by inhalation. |
|
Clinical Use |
Corticosteroid:Cerebral oedemaBacterial meningitis (unlicensed indication)Suppression of inflammatory and allergic disordersRheumatic diseaseCongenital adrenal hyperplasiaAnti-emetic (unlicensed indication) |
|
Safety Profile |
Poison by intraperitoneal and subcutaneous routes. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of F-. |
|
Synthesis |
Dexamethasone, 9α-fluoro-16α-methyl-11β,17,21-trihydroxypregna- 1,4-dien-3,20-dione (27.1.51), or simply 9α-fluoro-16α-methylprednisolone. The distinctive characteristic of dexamethasone is the presence of a fluorine atom at C9 of the steroid ring. Dexamethasone is synthesized in a multistage process from 3α-acetoxy-16-pregnen- 11,20-dione, which is reacted with methylmagnesium bromide in the presence of lithium bromide to give 3α-hydroxy-16α-methylpregnan-11,20-dione (27.1.39), after which a 17α- hydroxyl group is added. This is done by a reaction with acetic anhydride in the presence of p-toluenesulfonic acid, forming the 3-acetoxy-17-enolacetate 27.1.40, which is epoxidized by perbenzoic acid 27.1.41, and the product is hydrolyzed by an alkali to give an oxyketone 27.1.42. Addition of another hydroxyl group at C21 is accomplished by subsequent bromination of a methyl group with molecular bromine, replacing the bromine atom with iodine, and reacting iodide with potassium acetate, which forms the corresponding acetoxyketone 27.1.43. The hydroxyl group at C3 is oxidized to a carbonyl by chromium(VI) oxide in pyridine, giving the 3,11,20-triketone 27.1.44, which again undergoes bromination by molecular bromine, but at position C4. Dehydrogenation of this compound is accomplished using semicarbazide, which results in the formation of an unsaturated triketone 27.1.45. In order to avoid formation of semicarbazones at the keto-groups at C3 and C20, the final product is treated with pyruvic acid. Semicarbazones are then specially formed at the keto-groups of C3 and C20, and the keto-group at C11 that does not take part in semicarbazone formation is reduced to hydroxyl group using sodium borohydride. After removing the protective semicarbzone groups, 21-O-acetoxy-16β-methylhydrocortisone (27.1.46) is formed. This is reacted with potassium acetate and transformed to the epoxide 27.1.49. Reacting this with hydrofluoric acid results in an opening of the epoxide ring, during which the fluorohydrin 27.1.50 is formed. Finally, microbiological dehydrogenation of this compound at C1–C2 and simultaneous deacetylation gives dexamethasone (27.1.51). |
|
Veterinary Drugs and Treatments |
Glucocorticoids have been used in an attempt to treat practically every malady that afflicts man or animal, but there are three broad uses and dosage ranges for use of these agents. 1) Replacement of glucocorticoid activity in patients with adrenal insufficiency, 2) as an antiinflammatory agent, and 3) as an immunosuppressive. Among some of the uses for glucocorticoids include treatment of: endocrine conditions (e.g., adrenal insufficiency), rheumatic diseases (e.g., rheumatoid arthritis), collagen diseases (e.g., systemic lupus), allergic states, respiratory diseases (e.g., asthma), dermatologic diseases (e.g., pemphigus, allergic dermatoses), hematologic disorders (e.g., thrombocytopenias, autoimmune hemolytic anemias), neoplasias, nervous system disorders (increased CSF pressure), GI diseases (e.g., ulcerative colitis exacerbations), and renal diseases (e.g., nephrotic syndrome). Some glucocorticoids are used topically in the eye and skin for various conditions or are injected intra-articularly or intralesionally. The above listing is certainly not complete. For specific dosages and indications refer to the Doses section. High dose dexamethasone use for shock or CNS trauma is controversial; recent studies have not demonstrated significant benefit and it actually may cause increased deleterious effects. |
|
Drug interactions |
Potentially hazardous interactions with other drugsAldesleukin: avoid concomitant use.Antibacterials: metabolism accelerated by rifamycins; metabolism possibly inhibited by erythromycin; concentration of isoniazid possibly reduced.Anticoagulants: efficacy of coumarins and phenindione may be altered.Antiepileptics: metabolism accelerated by carbamazepine, fosphenytoin, phenobarbital, phenytoin and primidoneAntifungals: increased risk of hypokalaemia with amphotericin - avoid; metabolism possibly inhibited by itraconazole and ketoconazole; caspofungin concentration possibly reduced (may need to increase dose).Antivirals: concentration of indinavir, lopinavir, saquinavir and telaprevir possibly reduced; avoid with rilpivirine; concentration possibly increased by ritonavir.Ciclosporin: rare reports of convulsions in patients on ciclosporin and high-dose corticosteroids.Cobicistat: concentration possibly increased by cobicistat.Cytotoxics: possibly decreases axitinib concentration, increase dose of axitinib.Diuretics: enhanced hypokalaemic effects of acetazolamide, loop diuretics and thiazide diuretics.Netupitant: concentration of dexamethasone increased - halve dexamethasone dose.Vaccines: high dose corticosteroids can impair immune response to vaccines; avoid concomitant use with live vaccines. |
|
Metabolism |
Corticosteroids are metabolised mainly in the liver but also in other tissues, and are excreted in the urine. The slower metabolism of the synthetic corticosteroids with their lower protein-binding affinity may account for their increased potency compared with the natural corticosteroids. Up to 65% of a dose of dexamethasone is excreted in urine within 24 hours. |
|
Purification Methods |
Dexamethasone has been recrystallised from Et2O or small volumes of EtOAc. Its solubility in H2O is 10 mg/100mL at 25o; and is freely soluble in Me2CO, EtOH and CHCl3. [Arth et al. J Am Chem Soc 80 3161 1958; for the -methyl isomer see Taub et al. J Am Chem Soc 82 4025 1960, see Beilstein 8 IV 3501.] |
|
Who Evaluation |
Evaluation year: 2008 |
InChI:InChI=1/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15?,16+,17+,19+,20+,21+,22+/m1/s1
50-02-2 Relevant articles
Antigen-Drug Conjugates as a Novel Therapeutic Class for the Treatment of Antigen-Specific Autoimmune Disorders
Pickens, Chad J.,Christopher, Matthew A.,Leon, Martin A.,Pressnall, Melissa M.,Johnson, Stephanie N.,Thati, Sharadvi,Sullivan, Bradley P.,Berkland, Cory
, p. 2452 - 2461 (2019)
Multiple sclerosis represents the world'...
Method for recycling betamethasone or dexamethasone synthetic mother liquor materials
-
, (2020/03/12)
The invention relates to a method for re...
Synthesis method and application of 9-fluorosteroid compound
-
Paragraph 0089-0096; 0134-0136, (2021/01/15)
The invention provides a synthesis metho...
A 17, 21 - double-hydroxy steroid derivatives of synthetic method
-
Paragraph 0067; 0068; 0069; 0084, (2018/04/02)
The invention relates to a method of pre...
50-02-2 Process route
-
-
C23H31FO4

-
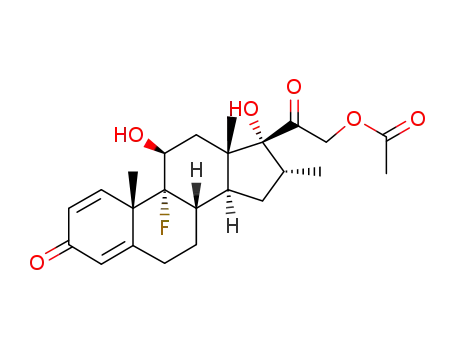
-
50-02-2
dexamethasone
| Conditions | Yield |
|---|---|
|
C23H31FO4;
In
ethyl acetate;
at 0 - 10 ℃;
With
hydrogenchloride;
In
water;
at 30 - 35 ℃;
for 1h;
|
40% |
-
-
1177-87-3
betamethasone

-

-
50-02-2
dexamethasone
| Conditions | Yield |
|---|---|
|
In
ethanol;
at 24 - 26 ℃;
for 96h;
Penicillium decumbens ATCC 10436, potato dextrose broth;
|
5% |
|
With
methanol; sodium carbonate;
at 20 ℃;
for 0.166667h;
Temperature;
|
50-02-2 Upstream products
-
1966-25-2
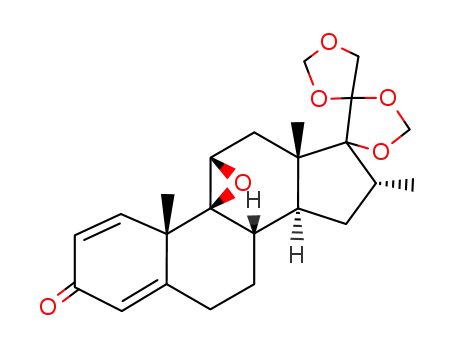
16α-methyl-9β,11β-oxido-17α,20;20,21-bismethylenedioxy-pregna-1,4-diene-3-one
-
1177-87-3

betamethasone
-
13209-41-1
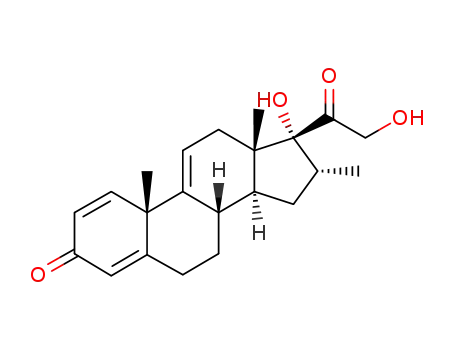
(8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one
-
14518-56-0
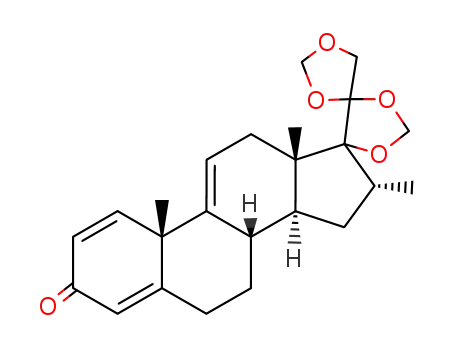
16α-methyl-17α,20;20,21-bismethylenedioxypregn-1,4,9(11)-triene-3-one
50-02-2 Downstream products
-
2265-22-7
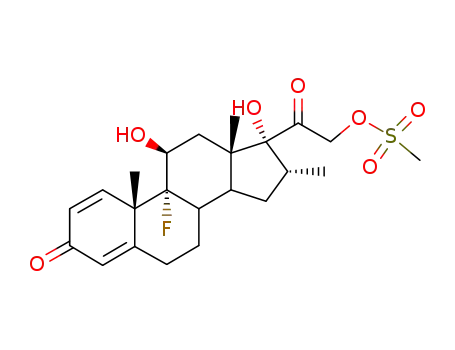
dexamethasone-21-mesylate
-
25122-41-2
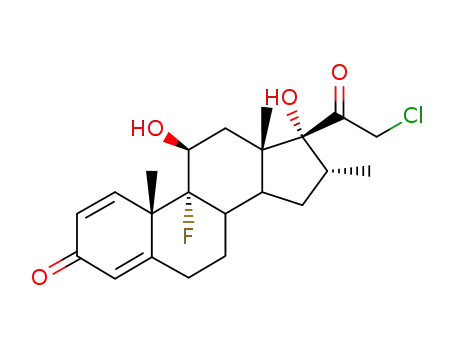
dexamethasone-21-chloride
-
1177-87-3
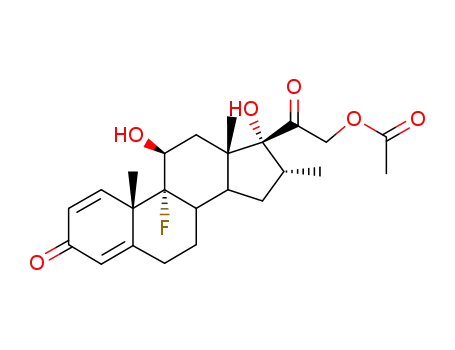
Dexamethasonacetat
-
37927-01-8
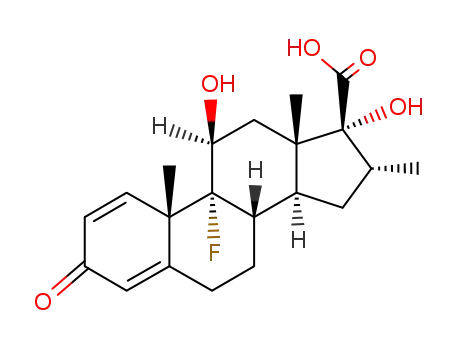
(8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthrene-17-carboxylic acid
Relevant Products
-
OSTARINE(MK-2866)
CAS:841205-47-8
-
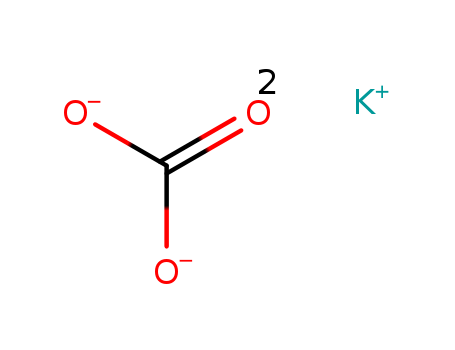
Potassium carbonate
CAS:584-08-7
-
2-Bromo-3'4'-(methylenedioxy)propiophenone
CAS:52190-28-0