98977-36-7
- Product Name:1-Boc-3-piperidone
- Molecular Formula:C10H17NO3
- Purity:99%
- Molecular Weight:199.25
Product Details;
CasNo: 98977-36-7
Molecular Formula: C10H17NO3
Appearance: white to yellow low melting solid
Factory Sells 1-Boc-3-piperidone 98977-36-7 Factory Supply with Lowest Price
- Molecular Formula:C10H17NO3
- Molecular Weight:199.25
- Appearance/Colour:white to yellow low melting solid
- Vapor Pressure:0.002mmHg at 25°C
- Melting Point:35-40 °C(lit.)
- Refractive Index:1.481
- Boiling Point:289.825 °C at 760 mmHg
- PKA:-1.71±0.20(Predicted)
- Flash Point:129.082 °C
- PSA:46.61000
- Density:1.099 g/cm3
- LogP:1.52430
1-Boc-3-piperidone(Cas 98977-36-7) Usage
|
Chemical Properties |
White to yellow low melting solid |
|
Uses |
1-Boc-3-piperidone, is used as an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff. |
InChI:InChI=1/C10H17NO3/c1-10(2,3)14-9(13)11-6-4-5-8(12)7-11/h4-7H2,1-3H3
98977-36-7 Relevant articles
Industrial adaptability selection for a novel ω-transaminase
Wang J 1 , Xie Y 1 , Wang H 1 , Wei D 1
, Chinese Journal of Biotechnology, 01 Sep 2020, 36(9):1929-1938
The strain could convert 20 mmol/L 1-Boc-3-pyrrolidinone and 20 mmol/L 1-Boc-3-piperidone with 85.84% and 67.42% conversion rate, respectively, in a 1-mL scale with isopropylamine (IPA) as amine donor.
Preparation method of N-tert-butyloxycarbonyl-3-piperidone and derivative thereof
-
Paragraph 0061-0065; 0067-0068; 0071, (2020/07/21)
The invention discloses a preparation me...
A pharmaceutical intermediate N - BOC - 3 - piperidone preparation method (by machine translation)
-
Paragraph 0055; 0059-0061; 0076-0077, (2018/03/01)
The invention belongs to the field of or...
An investigation into the role of 2,6-lutidine as an additive for the RuCl3-NaIO4 mediated oxidative cleavage of olefins to ketones
Watson, David W.,Gill, Matthew,Kemmitt, Paul,Lamont, Scott G.,Popescu, Mihai V.,Simpson, Iain
supporting information, p. 4479 - 4482 (2018/11/23)
2,6-Lutidine has been identified as a be...
98977-36-7 Process route
-
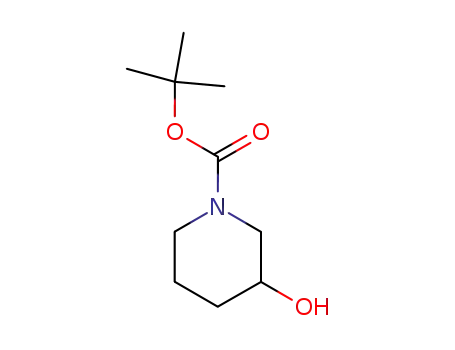
- 85275-45-2
N-tert-butoxycarbonyl-3-piperidinol

-
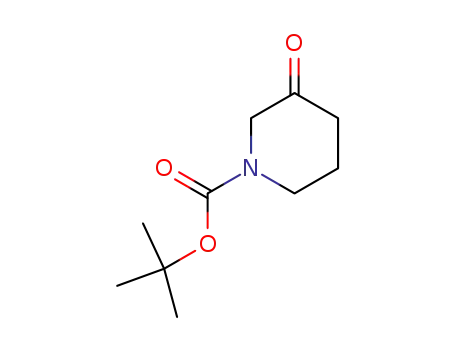
- 98977-36-7
3-oxo-piperidine-1-carboxylic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With sodium hypochlorite solution; sodium hydrogencarbonate; potassium bromide; In dichloromethane; at 5 - 10 ℃; pH=8.5; Reagent/catalyst;
|
99.4% |
|
With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium bromide; In dichloromethane; at 0 - 10 ℃; for 1h;
|
98.3% |
|
With Dess-Martin periodane; In dichloromethane; at 20 ℃; for 22h; Inert atmosphere;
|
97% |
|
With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate; sodium bromide; In diethyl ether; water; toluene; at 0 - 4 ℃;
|
95% |
|
With Dess-Martin periodane; In dichloromethane; at 0 - 20 ℃; for 18h;
|
86% |
|
With 4-methylmorpholine N-oxide; tetrapropylammonium perruthennate; In dichloromethane; at 0 - 20 ℃; for 1.33333h;
|
75% |
|
With pyridinium chlorochromate; In dichloromethane; at 20 ℃; for 3.25h;
|
19% |
|
With pyridine; chromium(III) oxide; acetic anhydride; In dichloromethane; Yield given;
|
|
|
With pyridine-SO3 complex; triethylamine; In dichloromethane;
|
|
|
With N-methyl-2-indolinone; tetrapropylammonium perruthennate; 3 A molecular sieve; In dichloromethane; for 1h;
|
|
|
With oxalyl dichloride; dimethyl sulfoxide; triethylamine; In dichloromethane; at -70 ℃;
|
|
|
With N-methyl-2-indolinone; tetrapropylammonium perruthennate; 4 A molecular sieve; In dichloromethane; acetonitrile; for 1h;
|
|
|
With trimethyl-sulfo-ammonium betaine; dimethyl sulfoxide; triethylamine; at 20 ℃; for 18h;
|
15.6 g |
|
With trioxide-trimethylamine; triethylamine; In dimethyl sulfoxide; at 20 ℃; for 18h;
|
15.6 g |
|
With trimethyl-sulfo-ammonium betaine; dimethyl sulfoxide; triethylamine; at 20 ℃; for 18h;
|
|
|
Multi-step reaction with 2 steps
1: (COCl)2 / CH2Cl2 / 1 h / -70 °C
2: Et3N / CH2Cl2 / 1 h / 0 °C
With oxalyl dichloride; triethylamine; In dichloromethane; 1: Swern oxidation / 2: Swern oxidation;
|
|
|
With triethylamine; In dimethyl sulfoxide; for 2h;
|
|
|
With pyridinium chlorochromate; In dichloromethane;
|
|
|
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; [bis(acetoxy)iodo]benzene; In dichloromethane; at 20 ℃;
|
|
|
With oxalyl dichloride; dimethyl sulfoxide; triethylamine; at 20 ℃; for 10h;
|
|
|
With sulfur trioxide trimethylamine complex; triethylamine; In dimethyl sulfoxide; at 20 ℃; for 18h;
|
|
|
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium; sode de l'acide trichloroisocyanurique; sodium bromide; In dichloromethane; water; at 5 ℃; for 4h; Reagent/catalyst; Time; Temperature;
|
-
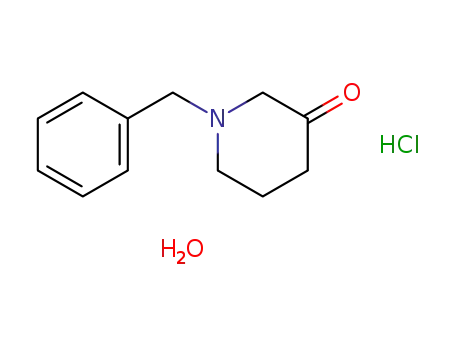
-
1-benzyl-piperidin-3-one hydrochloride hydrate

-

- 98977-36-7
3-oxo-piperidine-1-carboxylic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With hydrogen; palladium dihydroxide; In methanol; dichloromethane;
|
97% |
98977-36-7 Upstream products
-
24424-99-5
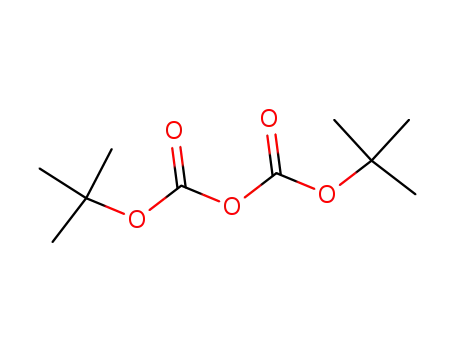
di-tert-butyl dicarbonate
-
50717-82-3
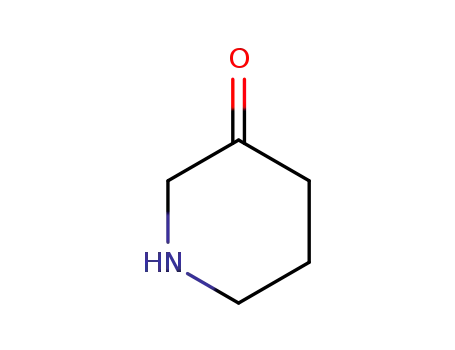
piperidin-3-one
-
85275-45-2

N-tert-butoxycarbonyl-3-piperidinol
-
6859-99-0
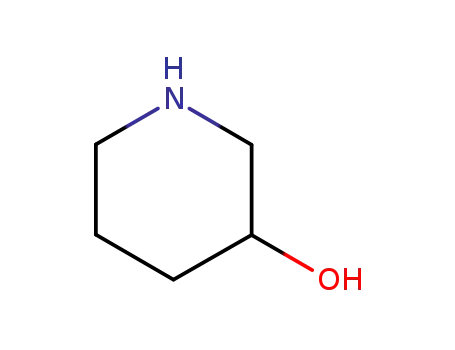
3-hydroxypiperazine
98977-36-7 Downstream products
-
98977-39-0
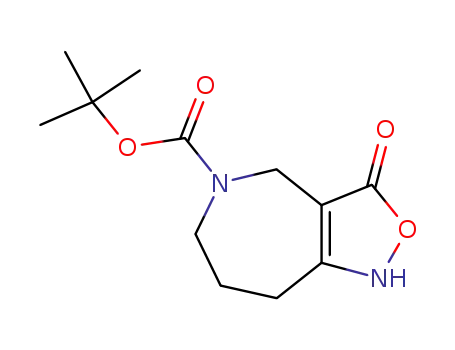
tert-butyl 1,3,5,6,7,8-hexahydro-3-oxo-4H-isoxazolo<4,3-c>azepine-5-carboxylate
-
98977-40-3
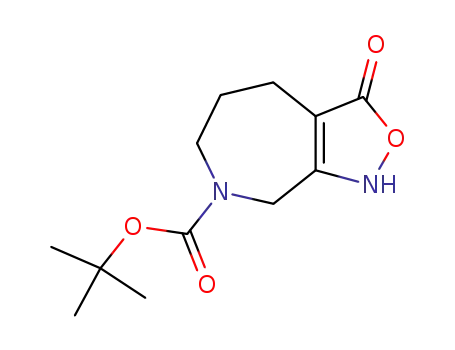
tert-butyl 1,3,5,6,7,8-hexahydro-3-oxo-4H-isoxazolo<4,3-c>azepine-7-carboxylate
-
98977-41-4
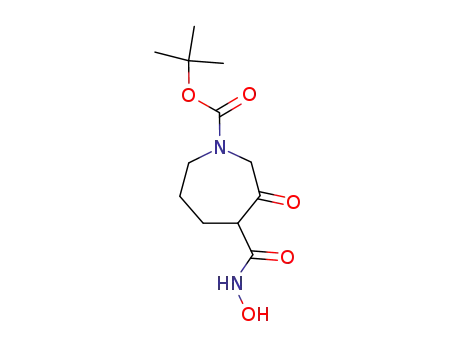
1-(tert-butyloxycarbonyl)-3-oxoperhydroazepine-4-hydroxamic acid
-
98977-37-8
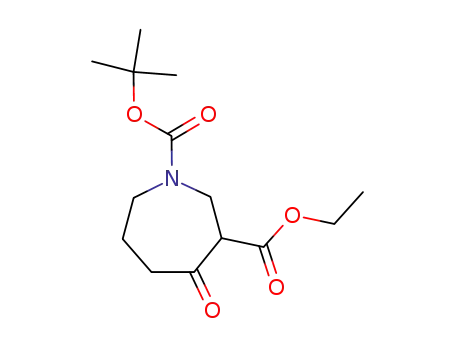
ethyl (+/-)-1-(tert-butyloxycarbonyl)-4-oxoperhydroazepine-3-carboxylate
Relevant Products
-
2, 5-dimethoxy-β-nitrostyrene
CAS:40276-11-7
-
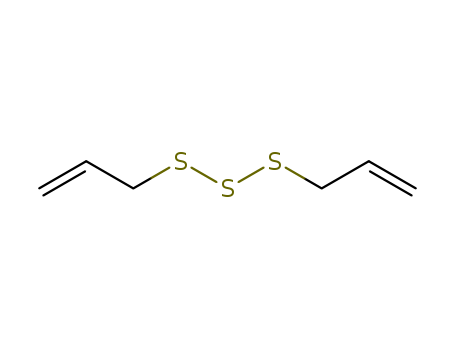
Diallyl trisulfide
CAS:2050-87-5
-
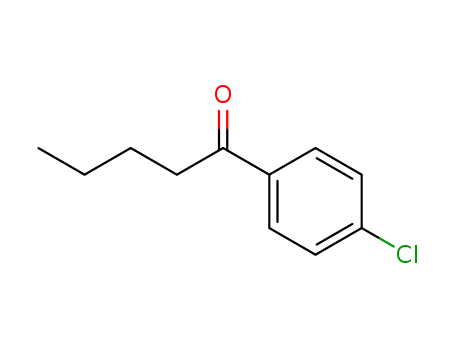
4-Chlorovalerophenone
CAS:25017-08-7