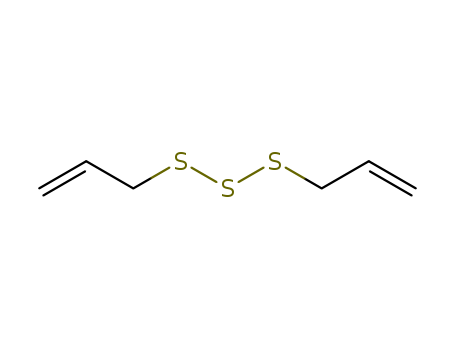
2050-87-5
- Product Name:Diallyl trisulfide
- Molecular Formula:C6H10S3
- Purity:99%
- Molecular Weight:178.343
Product Details;
CasNo: 2050-87-5
Molecular Formula: C6H10S3
Factory Supply 99% Pure Diallyl trisulfide 2050-87-5 Safe Shipping
- Molecular Formula:C6H10S3
- Molecular Weight:178.343
- Vapor Pressure:0.105mmHg at 25°C
- Refractive Index:1.581
- Boiling Point:229.5 °C at 760 mmHg
- Flash Point:87.8 °C
- PSA:75.90000
- Density:1.116 g/cm3
- LogP:3.38800
- IDLH:587
- IDLH:486
- IDLH:3265
Diallyl trisulfide(Cas 2050-87-5) Usage
|
Description |
Hydrogen sulfide (H2S) is an endogenously-produced gaseous second messenger that can regulate many physiological processes. Diallyl trisulfide (DATS) is an organic polysulfide compound found in garlic that acts as an H2S donor. It reduces the survival of prostate cancer PC-3 cells (IC50 = 22 μM) and inhibits the growth of human colon adenocarcinoma HCT15 cells (IC50 = 11.5 μM). DATS suppresses the growth of PC-3 xenografts in vivo in mice and induces vascular smooth muscle relaxation. Garlic extracts also lower cholesterol and there is evidence that DATS can alter the expression of genes and inhibit enzymes that are relevant to cholesterol synthesis. Diallyl trisulfide (DATS) is one of the organosulfur compounds found in garlic (Allium sativum), and other Allium genera such as onions. This sulfur-rich compound is produced by the rapid disintegration of its metabolic precursor Allicin. |
|
Chemical Properties |
Diallyl trisulfide has a disagreeable odor. It is a stable transformation product of allicin. Reported found in garlic oil. |
|
Uses |
Diallyl trisulfide (DATS) has been used to study its antimicrobial effects against Campylobacter jejuni. It has also been used to test its effect on hepatic regeneration in partial hepatectomy (PHx) rats. |
|
Definition |
ChEBI: An organic trisulfide that is trisulfane in which both of the hydrogens are replaced by allyl groups. A component of the essential oil of garlic and a major component of the traditional Chinese medicine allitridium, it exhibits antifungal, antitumour and a tioxidant activity |
|
Preparation |
In nature, diallyl trisulfide is formed from alliin and allicin. |
|
Biochem/physiol Actions |
Diallyl trisulfide (DATS) is a potent anti-cancer and chemopreventive agent that regulates apoptosis, metastasis, cell cycle, and angiogenesis in cancer pathways. It is also an excellent neuroprotective agent. DATS elicits antioxidant and anti-inflammatory activities against doxorubicin (DOX)-induced rats brains. It may improve glycemic control, muscle glucose utilization, and insulin secretion in streptozotocin-induced diabetic rats. |
|
Who Evaluation |
Evaluation year: 1999 |
InChI:InChI=1/C6H10S3/c1-3-5-7-9-8-6-4-2/h3-4H,1-2,5-6H2
2050-87-5 Relevant articles
Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds
Anna A. Powolny, Shivendra V. Singh
, Cancer Letters Volume 269, Issue 2, 8 October 2008, Pages 305-314
Allium vegetables, such as garlic, have been used for medicinal purposes throughout the recorded history. The known health benefits of Allium vegetables constituents include cardiovascular effects, improvement of the immune function, lowering of blood glucose level, radioprotection, protection against microbial infections, and anti-cancer effects.
3,4-Dichloro-1,2,5-thiadiazole: a commercially available electrophilic sulfur transfer agent and safe resource of ethanedinitrile
Gorjian, Hayedeh,Khaligh, Nader Ghaffari
, (2021/11/04)
3,4-Dichloro-1,2,5-thiadiazole is introd...
Selectivity of diallyl trisulfides (DATS) in reducing HAuCl4 to produce gold nanoparticles: a detailed investigation
Chatterjee, Arunavo,Mandal, Niladri Sekhar,Purkayastha, Pradipta
, (2021/09/08)
The bulbous root garlic (Allium sativum)...
Trisulfides over disulfides: Highly selective synthetic strategies, anti-proliferative activities and sustained H2S release profiles
Bhattacherjee, Debojit,Sufian, Abu,Mahato, Sulendar K.,Begum, Samiyara,Banerjee, Kaustav,De, Sharmistha,Srivastava, Hemant Kumar,Bhabak, Krishna P.
supporting information, p. 13534 - 13537 (2019/11/14)
Temperature-and solvent-induced selectiv...
2050-87-5 Process route
-
- 539-86-6
allicin

-
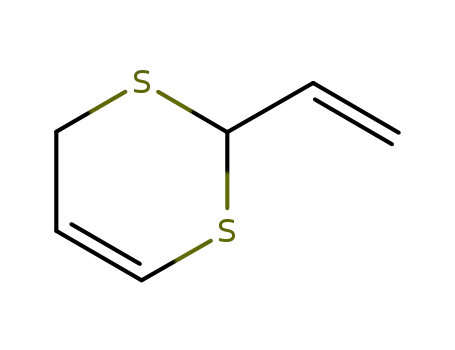
- 62488-53-3
3-vinyl-4H-<1,2>-dithiin

-
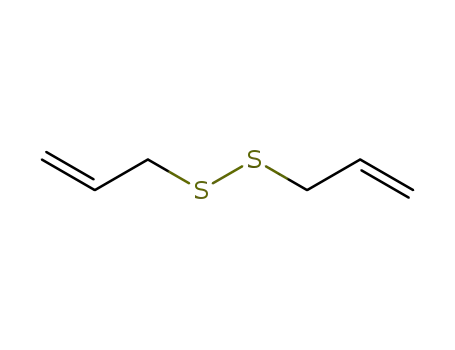
- 2179-57-9
diallyl disulphide

-
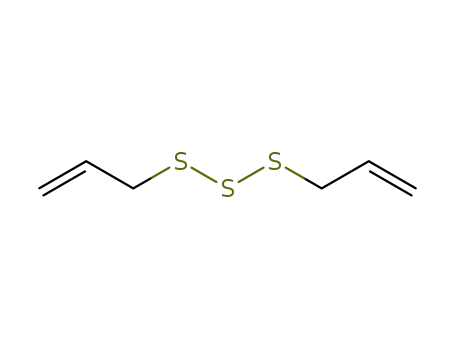
- 2050-87-5
diallyl trisulfide

-
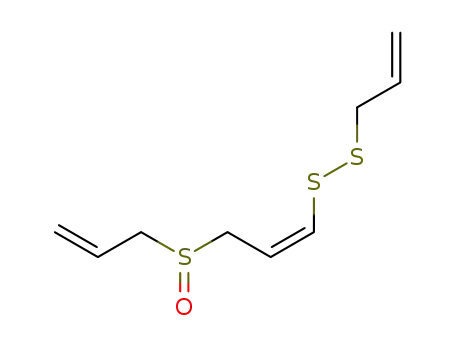
- 80028-57-5
2‐ethenyl‐4H‐1,3‐dithiine

-
- 92285-00-2
(Z)-ajoene

-
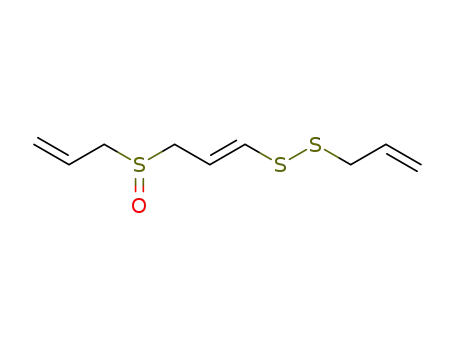
- 92284-99-6
(E)-ajoene
| Conditions | Yield |
|---|---|
|
at 37 ℃; for 48h; Product distribution; other temperatures, other times;
|
-
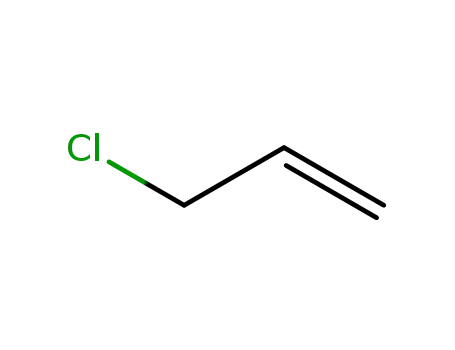
- 107-05-1
3-chloroprop-1-ene

-

- 2179-57-9
diallyl disulphide

-

- 592-88-1
diallyl sulphide

-

- 2050-87-5
diallyl trisulfide

-
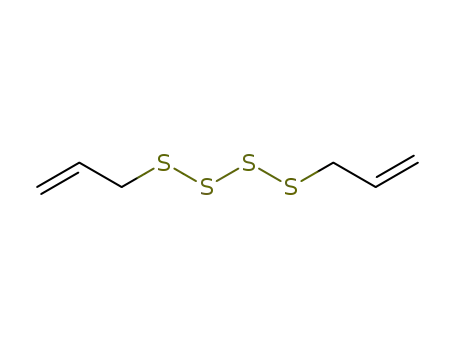
- 2444-49-7
diallyl tetrasulfide
| Conditions | Yield |
|---|---|
|
With sodium sulfide; sodium hydroxide; sulfur; polyethylene glycol; In water; at 45 - 60 ℃; under 1173.78 Torr;
3-chloroprop-1-ene; In water; at 45 - 50 ℃; under 915.2 - 4897.34 Torr; Product distribution / selectivity; continuous process;
|
|
|
With sodium sulfide; sodium hydroxide; sulfur; In water; at 45 - 60 ℃; under 1173.78 Torr;
3-chloroprop-1-ene; In water; at 45 - 50 ℃; under 915.2 - 4897.34 Torr; Product distribution / selectivity; continuous process;
|
2050-87-5 Upstream products
-
107-05-1
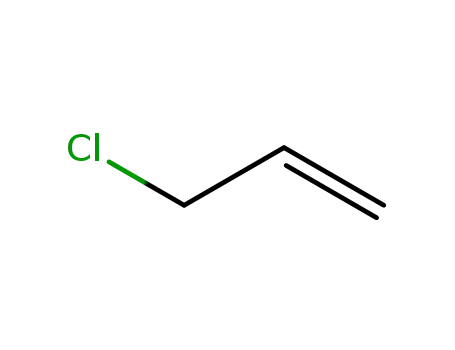
3-chloroprop-1-ene
-
870-23-5
prop-2-ene-1-thiol
-
2179-57-9

diallyl disulphide
-
539-86-6

allicin
2050-87-5 Downstream products
-
7783-06-4
hydrogen sulfide
-
27025-41-8
Oxidized glutathione
-
592-88-1

diallyl sulphide
Relevant Products
-
Tesamorelin
CAS:901758-09-6
-
Remdesivir Intermediate
CAS:1354823-36-1
-
1-Boc-3-piperidone
CAS:98977-36-7