224785-90-4
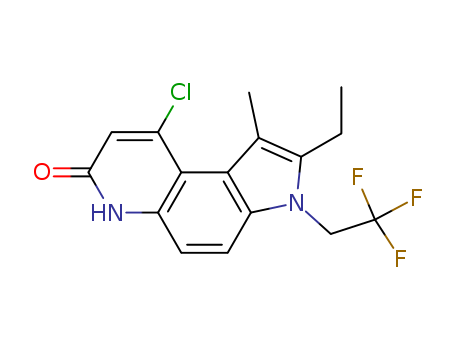
- Product Name:VardenafiL
- Molecular Formula:C23H32N6O4S
- Purity:99%
- Molecular Weight:488.611
Product Details;
CasNo: 224785-90-4
Molecular Formula: C23H32N6O4S
Appearance: White crystalline powder
Factory Supply High Purity Top Purity 99% VardenafiL 224785-90-4 on hot selling
- Molecular Formula:C23H32N6O4S
- Molecular Weight:488.611
- Appearance/Colour:White crystalline powder
- Vapor Pressure:5.17E-19mmHg at 25°C
- Melting Point:230-235 °C
- Boiling Point:692.2 °C at 760 mmHg
- PKA:9.86±0.20(Predicted)
- Flash Point:372.5 °C
- PSA:121.28000
- Density:1.37 g/cm3
- LogP:4.63100
Vardenafil hydrochloride trihydrate(Cas 224785-90-4) Usage
|
Description |
Vardenafil is a new PDE5 inhibitor launched for oral treatment of male erectile dysfunction and it has significant structural similarity with sildenafil (Viagra?), which was the first PDE5 inhibitor introduced in 1998 for this indication. Vardenafil is synthesized in three steps starting with a cyclization reaction of 2-ethyoxybenzamidine with 2-butyramidopropionic acid and ethoxyallyl chloride to construct the imidazotriazine ring system, followed by sulfonation to the corresponding sulfonyl chloride and subsequent condensation with 1-ethylpiperazine. The potency of PDE5 inhibition by vardenafil (IC50=0.7 nM) is ~10 times greater than that of sildenafil (IC50=6.6 nM). Vardenafil is typically administered in single doses of 10 and 20 mg. The time to reach maximum plasma concentration is 0.75 h, which is slightly shorter than those of sildenafil (tmax=1.16 h) and tadalafil (tmax=2h), and the half-life is 4–5 h. Although it is almost completely absorbed following oral administration, the mean absolute bioavailability of a 10 mg dose is ~15%, resulting from extensive first pass metabolism. Vardenafil is metabolized in the liver primarily by CYP3A4 and is eliminated mainly in feces. In clinical studies, 10–20 mg doses of vardenafil was well tolerated and efficacious in patients with ED of various severities, including subjects with comorbidities such as diabetes mellitus or hypertension or hyperlipidemia. The side-effect profile of vardenafil is similar to that of sildenafil, with headache, flushing, dyspepsia and nasal congestion being the most common adverse events. Vardenafil has systemic vasodilatory properties, which can cause transient decrease in supine blood pressure; however, it does not appear to translate into clinical effects. The mean maximum decreases in supine systolic blood pressure following 20 and 40 mg vardenafil were 6.9 and 4.3 mmHg, respectively, when compared to placebo. However, single and multiple oral doses of vardenafil up to 40 mg produced no clinically relevant changes in the ECGs of normal male volunteers. |
|
Originator |
Bayer AG (Germany) |
|
Uses |
erectil dysfunction;PDE5 inhibitor |
|
Definition |
ChEBI: The sulfonamide resulting from formal condensation of the sulfo group of 4-ethoxy-3-(5-methyl-7-propylimidazo[5,1-f][1,2,4]triazin-4(1H)-one-2-yl)benzenesulfonic acid and the secondary amino group of 4-ethylpiperazine. |
|
Brand name |
Levitra |
|
Flammability and Explosibility |
Nonflammable |
|
Clinical Use |
Treatment of erectile dysfunction |
|
Drug interactions |
Potentially hazardous interactions with other drugs Alpha-blockers: enhanced hypotensive effect - avoid for 6 hours after alpha-blockers (max dose 5 mg). Antifungals: concentration increased by ketoconazole, and itraconazole - avoid concomitant use. Antivirals: concentration increased by fosamprenavir, indinavir and ritonavir- avoid with indinavir and ritonavir; increased risk of ventricular arrhythmias with saquinavir - avoid; avoid with telaprevir, use tipranavir with caution. Cobicistat: concentration of vardenafil possibly increased - reduce dose of vardenafil. Grapefruit juice: concentration possibly increased - avoid concomitant use. Nicorandil: possibly enhanced hypotensive effect - avoid concomitant use. Nitrates: possibly enhanced hypotensive effect - avoid concomitant use. Riociguat: enhanced hypotensive effect - avoid concomitant use. |
|
Metabolism |
Vardenafil is metabolised in the liver primarily by cytochrome P450 isoenzymes CYP3A4 (the major route) as well as CYP3A5 and CYP2C isoforms. The major metabolite produced by desethylation of vardenafil also has some activity. Vardenafil is excreted as metabolites mainly in the faeces (91 to 95%), and to a lesser extent in the urine. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C23H32N6O4S.ClH.3H2O/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28;;;;/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30);1H;3*1H2
224785-90-4 Relevant articles
A METHOD FOR THE PREPARATION AND ISOLATION OF SALTS OF VARDENAFIL WITH ACIDS
-
Page/Page column 17, (2013/06/06)
The subject of this invention provides a...
Synthesis and spectral characterization of related substances of vardenafil, an erectile dysfunction drug
Reddy, Vajrala Venkata,Naga Brahmeswara Rao, Mandava Venkata,Reddy, Ghanta Mahesh,Mukkanti, Khagga,Reddy, Ganta Madhusudhan
, p. 3513 - 3523 (2012/10/18)
Vardenafil hydrochloride trihydrate (Lev...
N-BUTYRAMIDE, THE PREPARATION METHOD AND USE THEREOF
-
, (2011/08/08)
Disclosed are N-{1-[3-(2-ethoxy-5-(4-eth...
A PROCESS FOR THE PREPARATION AND ISOLATION OF VARDENAFIL AND SALTS THEREOF
-
Page/Page column 11, (2011/07/30)
A new process for the preparation of var...
224785-90-4 Process route
-
-
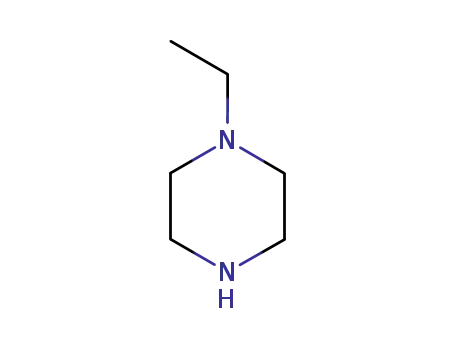
5308-25-8
4-ethylpiperazine

-
![2-(2-ethoxy-phenyl)-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-one](/upload/2023/9/289d5aa1-8f60-4617-951e-115b4de34f3f.png)
-
224789-21-3
2-(2-ethoxy-phenyl)-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-one

-
-
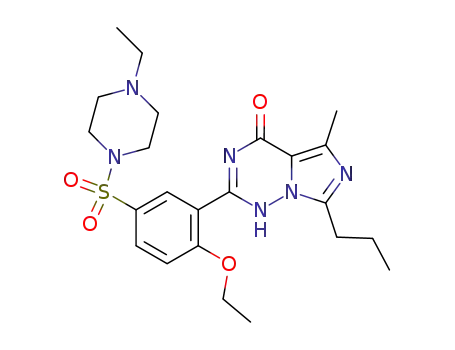
224785-90-4
vardenafil
| Conditions | Yield |
|---|---|
|
2-(2-ethoxy-phenyl)-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-one;
With
chlorosulfonic acid;
at 0 - 22 ℃;
for 0.75h;
4-ethylpiperazine;
In
dichloromethane;
at -3 - 25 ℃;
for 0.75h;
Product distribution / selectivity;
|
-

-
5308-25-8
4-ethylpiperazine

-
![4-ethoxy-3-(5-methyl-4-oxo-7-propyl-3,4-dihydroimidazo[5,1-f][1,2,4]triazin-2-yl)-benzenelsulfonic acid chloride](/upload/2023/9/aadceda4-b27c-4865-b754-246914193c41.png)
-
224789-26-8
4-ethoxy-3-(5-methyl-4-oxo-7-propyl-3,4-dihydroimidazo[5,1-f][1,2,4]triazin-2-yl)-benzenelsulfonic acid chloride

-

-
224785-90-4
vardenafil
| Conditions | Yield |
|---|---|
|
In
dichloromethane;
|
224785-90-4 Upstream products
-
5308-25-8

4-ethylpiperazine
-
224789-26-8
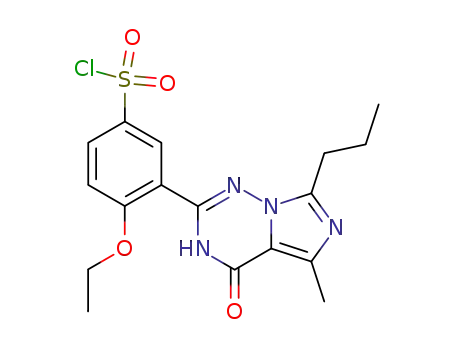
4-ethoxy-3-(5-methyl-4-oxo-7-propyl-3,4-dihydroimidazo[5,1-f][1,2,4]triazin-2-yl)-benzenelsulfonic acid chloride
-
437717-43-6
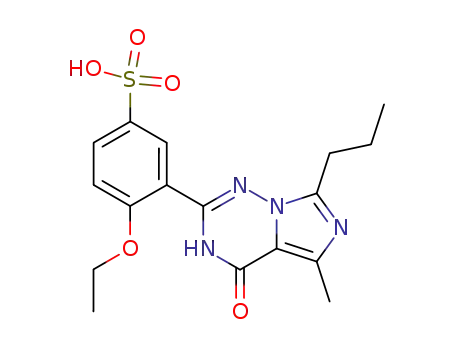
4-ethoxy-3-(5-methyl-4-oxo-7-propyl-3,4-dihydroimidazo[5,1-f][1,2,4]triazine-2-yl)phenylsulfonic acid
-
1169861-31-7
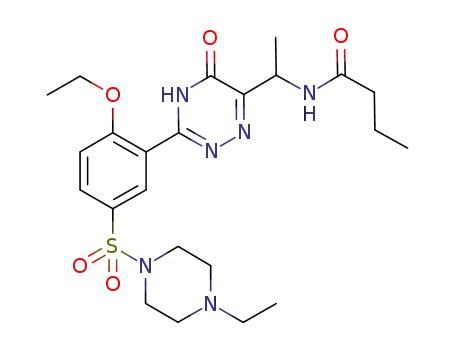
N-[1-[3-[2-ethoxy-5-[(4-ethyl-1-piperazinyl)sulfonyl]-phenyl]-2,5-dihydro-5-oxo-1,2,4-triazin-6-yl]ethyl]-butanamide
224785-90-4 Downstream products
-
448184-48-3
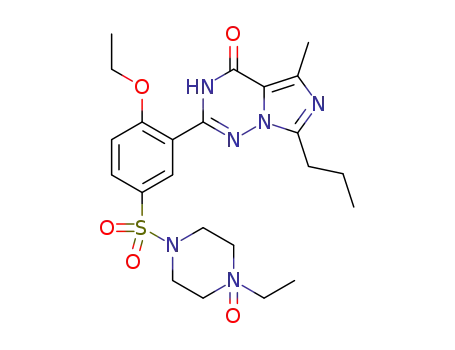
2-{2-ethoxy-5-[(4-ethyl-4-hydroxy-4λ5-piperazin-1-yl)sulfonyl]phenyl}-5-methyl-7-propyl imidazo-[5,1-f]-1,2,4-triazin-4(3H)-one N-oxide
-
437717-43-6

4-ethoxy-3-(5-methyl-4-oxo-7-propyl-3,4-dihydroimidazo[5,1-f][1,2,4]triazine-2-yl)phenylsulfonic acid
Relevant Products
-
Tesamorelin
CAS:901758-09-6
-
LGD-3303
CAS:917891-35-1
-
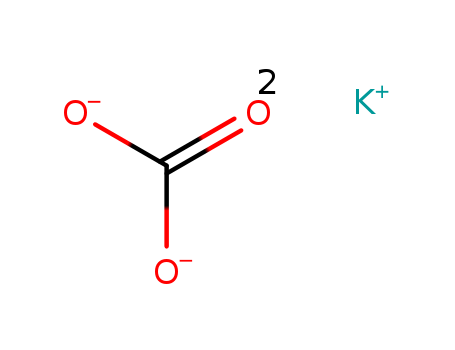
Potassium carbonate
CAS:584-08-7