901758-09-6
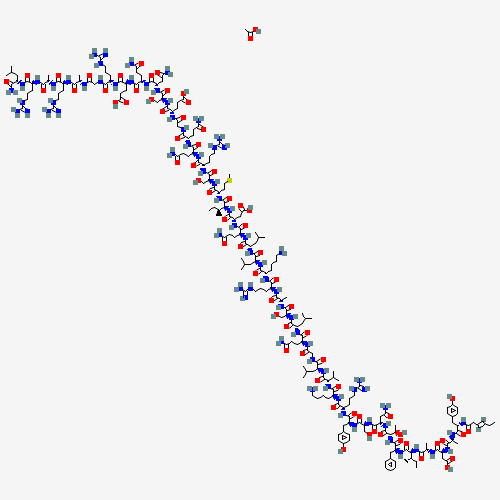
- Product Name:Tesamorelin
- Molecular Formula:C223H370N72O69S
- Purity:99%
- Molecular Weight:5196
Product Details;
CasNo: 901758-09-6
Molecular Formula: C223H370N72O69S
Buy High Grade Wholesale Tesamorelin 901758-09-6 with Low Price
- Molecular Formula:C223H370N72O69S
- Molecular Weight:5196
Tesamorelin(Cas 901758-09-6) Usage
|
Chemical Description |
Tesamorelin injection is a hormone similar to the one normally released from the hypothalamus gland in the brain. It is used to reduce excess fat (lipodystrophy) in the abdomen or stomach in patients infected with human immunodeficiency virus (HIV). |
|
Uses |
Tesamorelin injection is in a class of medications called human growth hormone-releasing factor (GRF) analogs. In the bodybuilding world, some folks have been using growth hormone-releasing peptides like Tesamorelin to boost muscle growth and reduce body fat potentially. These peptides can stimulate the growth hormone release, increasing Insulin-Like Growth Factor-1 (IGF-1) levels. Many people using this medication do not have serious side effects. This medicine may cause swelling (fluid retention) in some parts of your body. |
901758-09-6 Relevant articles
Tesamorelin, a human growth hormone releasing factor analogue
Ying Wang , PhD &Brian Tomlinson , MD FRCP
, Expert Opinion on Investigational Drugs Volume 18, 2009 - Issue 3
This article reviews the development of tesamorelin and its purported role in HIV-related lipodystrophy and other potential indications.
Effects of Tesamorelin, a Growth Hormone–Releasing Factor, in HIV-Infected Patients With Abdominal Fat Accumulation: A Randomized Placebo-Controlled Trial With a Safety Extension
Falutz, Julian MD*; Potvin, Diane MSc†; Mamputu, Jean-Claude PhD†; Assaad, Hani MD†; Zoltowska, Monika PhD†; Michaud, Sophie-Elise PhD†; Berger, Daniel MD‡; Somero, Michael MD§; Moyle, Graeme MD, MBBS‖; Brown, Stephen MD¶; Martorell, Claudia MD, MPH#; Turner, Ralph PhD, MPH**; Grinspoon, Steven MD†
, JAIDS Journal of Acquired Immune Deficiency Syndromes 53(3):p 311-322, March 1, 2010.
In the extension phase (months 6-12), patients receiving tesamorelin were rerandomized to continue on tesamorelin (2 mg SC every day) or switch to placebo. Patients initially randomized to placebo switched to tesamorelin.
Relevant Products
-
VardenafiL
CAS:224785-90-4
-
Ethylmagnesium bromide
CAS:925-90-6
-
1-Benzyl-3-piperidinol
CAS:14813-01-5